Reviews
Sebastian Torres
Curved Cannulas Cheekbone Augmentation with Novel Hyaluronic Acid Dermal filler: clinical outcomes and patient satisfaction
Keywords | Summary | Correspondence | References
Keywords
cheekbone augmentation, Curved cannulas, Filler, Hyaluronic acid
Schlüsselworte
Summary
Background: Cheekbone augmentation represents a common request among facial rejuvenation procedures, due to its impressive results in the midface volumetric lifting capacity. Objective: To evaluate the safety, efficacy and patient satisfaction with the use of a new cross-linked hyaluronic acid (HA) based dermal filler (Decoria Voluma, Bohus Biotech, Sweeden, EC) in augmenting zygomatic and malar region (Cheekbone) through a novel zygomatic curved cannula (Torres curved cannulas set, Notrox instruments, Pakistan). Materials and methods: This was a single center, blind evaluator, 300-day study in which 90 patients were treated at their baseline visit with up to five 1 mL syringes of HA. The majority of subjects were treated using curved cannulas. A small control group was treated with traditional straight cannulas, to analyze compliance differences. The physician and evaluator assessed patients, clinically and through 3D software, 7 days after treatment and then every month after the initial treatment for 10 months (300 days). Moreover, patient satisfaction was measured at 7d, 1, 3, 6 and 10m through a self-evaluation questionnaire. Results: Subjects experienced statistically significant improvement in Cheekbone projection and Ogee Curve and maintained those results for more than 240 days. In proximity of the end of the observational period (300 days) the studied area revealed minor reabsorption of the product being at all times better than baseline. Patient satisfaction scores were rather excellent or very good in the curved cannula group and good in the straight cannula group for all the length of the study. Conclusion: Injectable HA new cross-linked based dermal filler (Decoria Voluma, Bohus Biotech,Sweeden, EC) was efficacious in augmenting cheekbones, resulting in satisfactory corrections up to 300 days and excellent patient compliance and satisfaction rate. Treatments were better tolerated and scored higher satisfaction rates when performed through curved cannulas.
Zusammenfassung
Introduction
Dermal fillers are increasingly popular as an alternative for facial rejuvenation, being the second most common non-surgical cosmetic procedure with exponential growth in the last years [1]. Filler injections growth rate is about the same for patients regardless the gender [2]. Key features of filler treatment rely on patient compliance and satisfaction rate [3-7], generally measured through satisfaction questionnaires [8], and safety, efficacy and lasting effect of the corrections [9].
The zygomatic-malar region is defined by the intersection of the lines passing between the ocular lateral cantus and the oral commissure and tragus to nasal ala. It is considered the landmark of the midface and gives the face the main volumetric projection that allows the visualization of an oval face in frontal view. Typically, young faces present in three quarters view, a high and projected external “S shape” profile, known as Ogee curve [10], that outlines the zygomatic prominence (Fig. 1). The cheek has several multilevel fat pads and retaining ligaments [11]. Aging volumetric deflation and gravity contributes to folds accentuation and uneven contours.
Facial gender differences typically manifest in this region, being female cheekbones higher and more projected with soft transitions towards the inferior portion of the cheek [12].
Treating Cheekbones
For cheekbones, the difference between females and males is that in males the projection of the superior pole transits abruptly to no volume in the buccal area with a strong and define transition. Zygomatic and malar area are enhanced giving the aspect of a bony prominence with strong transition desirable to the rest of the soft tissues.
In females the augmentation of the cheekbones needs more volume and foresees a soft transition of volume to connect with the buccal fat pad. The ideal cheek has more volume superiorly with an apex that typically requires augmentation either laterally (white females) or medially (men, Asian females), with a smooth ogee curve [13].
Several dermal fillers have been used to enhance this area [14-15], being hyaluronic acid dermal fillers among the most popular due to their good safety and efficacy profile [16-17].
Several techniques are described, such as bolus o retrogradal linear techniques, with the aid of needles or cannulas. The former although more precise, results in more traumatic delivery, higher bruising, increased downtime and greater likelihood of vascular laceration or intravascular placement. Cannulas advocates claim less bruising and swelling and increased peri vascular/neural safety [18-19].

Fig. 1: Ogee curve. External S shaped curve that outlines the facial contour in three quarters view.
Curved cannulas, being a new medical device introduced in 2017 [20], lack of corresponding comparative studies with classic straight units, being this one of the first attempts to show its clinical benefits.
The author technique of choice is a stacking technique with curved cannulas, due to even filler distribution, lesser product consumption and better tissue projection.
The use of a new cross-linked hyaluronic acid (HA) based dermal filler (Decoria Voluma, Bohus Biotech, Sweeden, EC) in combination with the use of a novel zygomatic curved cannula [20] (Torres curved cannula set, Notrox Instruments, Pakistan) was tested for cheekbone volumetric enhancement, regarding patient satisfaction, safety, efficacy and lasting effect.

Fig. 3: Cheekbone Treatment Plan: Blue circle: Zygomatic-malar region (correction target). White discontinuous line: malar septum. Yellow stripes: curved cannula correction vectors, retrograde technique. Red circle: cannula entry point.
Materials and Methods
Eligible participants were women aged 18 and older seeking tissue augmentation treatments for the Cheekbones, that came to the Clinic between January and October 2020. After local ethics committee approval, the procedure and study design were discussed with patients and informed consents were obtained.
Exclusion criteria included poor general health, known hypersensitivity or allergy to the treatment components, breastfeeding or pregnancy, previous permanent fillers treatments in the area, or temporal fillers in the area in the previous 10 months. Other exclusion criteria included; history of autoimmune diseases; active skin disease, irritation, or inflammation in the target areas of injection.
A new cross-linked hyaluronic acid (HA) based dermal filler (Decoria Voluma, Bohus Biotech, Sweeden, EC) was used in combination with a novel zygomatic curved cannula (Torres curved cannula set, Notrox Instruments, Pakistan – Fig. 2). The syringes contain 1mL of cross-linked HA, the maximum volume per patient did not exceed 5 ml.
Ninety evaluable patients with moderate midface volume depletion or cheekbone enhancement wishes, who met all study inclusion and lack exclusion criteria were enrolled into this single center, evaluator-masked, study.
Each subject underwent one treatment with up to five 1mL syringes of HA. Each HA syringe was attached to a 5 cm x 1 mm curved cannula in preparation for injection. A control group of 15 individuals was treated with a 5 cm x 1 mm straight cannula. The same physician treated all patients in a similar manner. The area to be treated was properly cleansed with chlorhexidine. The midpoint of the nasolabial fold was anesthetized with a small bleb of local anesthetic, and a 21 G needle was used to penetrate the skin to allow cannula entry. HA was deposited in the deep subcutaneous plane covering all the zygomatic-malar region using a linear retrograde technique; directed straight along the target area. Attention was given to interrupt the malar septum that divides zygomatic and malar fat to grant an even distribution of the filler. The patients were asked to smile during the procedure to reveal muscular action and points of structural breakdown. Extra material was delivered perpendicular to these areas. The treatment design is shown in Figure 3.
Any skin blebs were massaged down after administration. Total product administered varied per patient based on patient wishes, with most patients receiving an average of 2 mL (~2 syringes) per treatment session. Total volume treatment was recorded. Patients followed up 7 days after treatment and then every 30 days after the initial treatment session for 300 days.
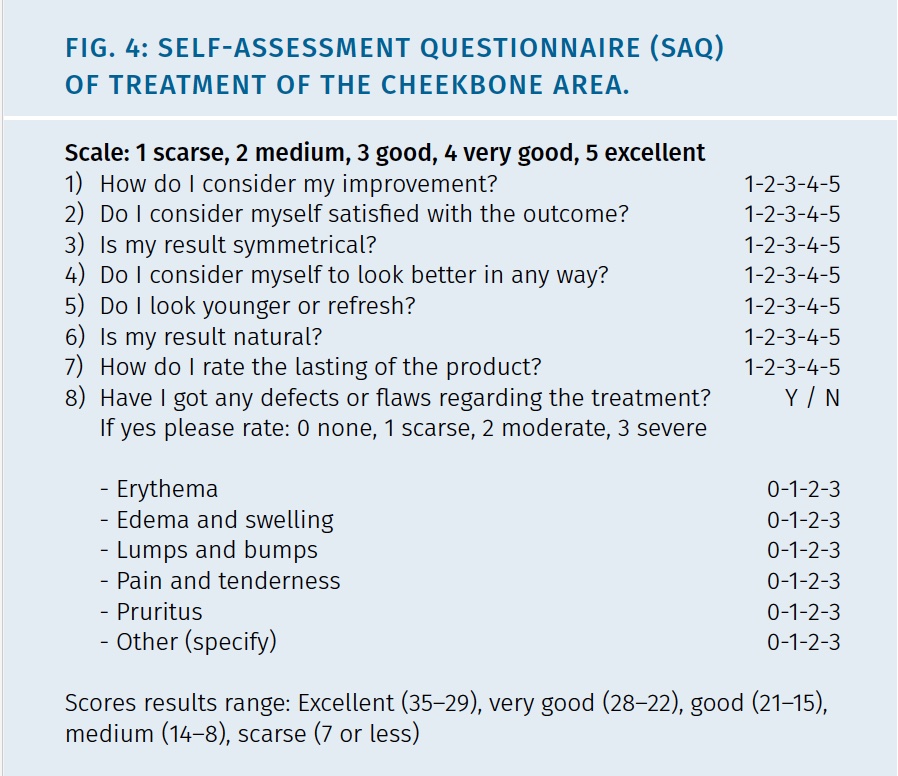
Self-assessment questionnaire (SAQ) were applied to patients at 7d, 1, 3, 6 and 10 months to evaluate compliance and satisfaction rate. Details of SAQ are show in Figure 4.
Outcome and Statistical Analysis
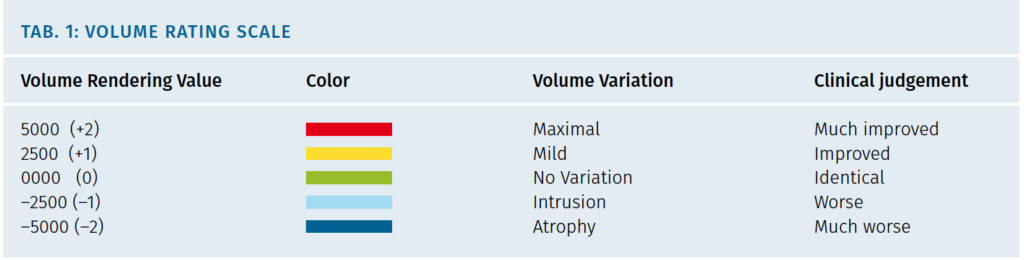
Standardized 3D imaging (Quantificare Life Viz mini, France, EC) were taken at each visit. Life Viz software (Quantificare, France, EC) was used to obtain volumetric baseline rendering and to compare volume variations in time at different controls. The blind observer assessed subjective clinical aesthetic improvement of the cheekbone area. Objective cheekbone volume variations were measure with Life Viz Software, in cm3 which gave an associated color. According to the volume-color variations in the scale the outcome was informed as: -2 much worse, -1 worse, 0 identical, +1 improved, +2 much improved (Fig. 5).
Participants completed four satisfaction questionnaires at 7d, 1, 3, 6 and 10 months after the treatments. The former, assess overall satisfaction considering the treatment area.
The questionnaire focused on the aesthetic results after treatment and contained 7 single-choice questions. For each single-choice question, a scale of 5 possible score options (scarse 1, medium 2, good 3, very good 4, excellent 5), was provided, so that participants had opportunities to provide their feedback regarding treatment. The SAQ scores were arbitrarily defined according to their range in: Excellent (35 – 29), very good (28 – 22), good (21 – 15), medium (14 – 8) or scarse (7 or less).
Adverse events (AEs) were monitored throughout the study. At each study visit, the investigators assessed erythema, edema and swelling, bruising, lumps and bumps, pain and tenderness, and pruritus on a scale of 0 (none) to 3 (severe). During the entire duration of the study patients recorded the possible adverse events and rate them using the same scale within the SAQ.

Fig. 6: Female 24y, Treatment of Cheekbone, Decoria Voluma, 4 ml total (2 ml per side). Curved cannula technique from NLF.
Statistical Analysis
Statistical analysis was done with excel 13 (windows 10). P .05 was considered to be statistically significant, and .001 was considered to be highly statistically significant.

Fig. 7: Female patient, 35y, Cheekbone Enhancement, Decoria Voluma, 3 ml total, Curved cannula technique.
Results
Ninety Hispanic American female patients were enrolled in the study. The mean age of the patients was 42 (range 18-55).
Seven patients were lost during the length of the study (4 straight and 3 curved cannula group). 83 patients completed the study (72 curved and 11 straight cannula group). The mean amount of HA injected for the cheekbone area was 2 mL, with a range from 1,5 – 5 mL.
Baseline cheekbone volume was considered as 0 of numeric value. Cheekbone projection improved a median 1,18-point scale by day 7 (p < .001) and remained statistically significantly improved by day 300 (p = .003), although by day 180, the level of improvement had begun to decrease. Media improvement for the whole period of study was 1.02 (7d = 1.18; 1m = 1.11; 3m = 1; 6m = 0.89; 10m = 0.78).
Satisfaction questionnaires was rated as very good or excellent for the majority of the controls for the curved cannula group at 7d (median 30.5), 1m (median 29.76), 3m (median 28.84), 6m (median 27.6) and 10m (median 26.8). The global median for all the study period in this group was 28,72.
Satisfaction rates in the straight cannula group were lower but still good at all times during the study (Overall media: 19 / 7d 20.1 / 1m 19 / 3m 18.3 / 6m 17.7 /10m 17.5).
Side effects included bruising 4.8% (n = 4; 3 straight cannula /1 curved cannula group), swelling 3.6% (n = 3; straight cannula group), bumpiness 2.4% (n = 2 straight cannula group), asymmetry 1.2% (n = 1 straight cannula group), and erythema/discoloration 1.2% (n = 1 straight cannula group). All above were self-limiting within the first 1 – 2 weeks post injection. Tyndall effect, granulomatous or nodular reactions, and focal necrosis were not registered.
Discussion and Conclusions
A successful filler treatment is defined as a good aesthetic result, free of complications, with a good evolution in time and maximal patient compliance and satisfaction [21]. The former is possible with the correct selection of the patient, material and technique [22-23].
A new cross-linked HA dermal filler (Decoria Voluma, Bohus Biotech, Sweeden, EC) probed to be effective in cheekbone rejuvenation/enhancement with consistent results, maintained during all along study length. Patients and physician satisfaction, was very good or excellent for the majority. Interestingly clinical subjective judgment was able to be correlated with objective 3D imaging software (Quantificare Life Viz, France, EC) to estimate corrections and evaluate their performance and lasting in time. Most subject were informed as having a mild cheekbone volume improvement, probably to software sensitivity to volume.
The face is an oval and as such is formed by curves. Following a curve with a straight instrument, such as traditional blunt tip cannulas, generally needs tissues compression causing patient discomfort and greater downtime. Curved cannulas allow to follow facial curves reducing tissue stress and inflammatory response, especially in the cheekbone area, which often needs greater volume enhancement than elsewhere in the face.
Satisfaction scores in SAQ were significantly higher in the curved cannula group, probably related to immediate treatment discomfort and swelling, higher in the straight cannula group. Although adverse events were few and self-limited they were higher in the straight cannula group which also experimented a higher associated patient loss, during the observational period.
Curved cannulas seem to have higher satisfaction rates for the treatment and less complications, although a bigger number of individuals should be studied to establish definitive tendencies.
Address of Correspondence
Sebastian Torres, MD
Plastic, Maxillofacial and Aesthetic Surgery
Lo Fontecilla 101, oficina 916, Las Condes
Santiago, Chile
sebastiantorresmd@gmail.com
Conflict of Interests
Dr. Torres is an international freelance medical advisor for Bohus Biotech, without any economical gain or contract. Dr. Torres is the inventor of the Torres curved cannula set, currently commercialized by Notrox, Instruments, Pakistan.
References
1. American Society for Aesthetic Plastic Surgery. Buttock Augmentation and Labiaplasty. Available at https://www.surgery.org/sites/default/files/ASAPS-Stats2018-Trends.pdf. 2019. Accessed December 15, 2019.
2. Frucht CS, Ortiz AE. Nonsurgical cosmetic procedures for men: trends and technique considerations. J Clin Aesthet Dermatol. 2016, 9(12): 33-43.
3. Fabi SG, Champagne JP, Nettar KD, Maas CS, Goldman MP. Efficacy and safety of and patient satisfaction with injectable hyaluronic acid with 0.3% lidocaine hydrochloride for the treatment of superficial perioral lines or superficial lateral canthal lines. Dermatol Surg. 2013; 39(11): 1613-20.
4. Rzany B, Cartier H, Kestemont P, Trevidic P, Sattler G, Kerrouche N, et al. Full‐Face rejuvenation using a range of hyaluronic acid fillers: efficacy, safety, and patient satisfaction over 6 months. Dermatol Surg. 2012; 38(7pt2): 1153-61.
5. Smith L, Cockerham K. Hyaluronic acid dermal fillers: can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration? Patient Prefer Adherence. 2011; 5: 133.
6. Buntrock H, Reuther T, Prager W, Kerscher M. Efficacy, safety, and patient satisfaction of a monophasic cohesive polydensified matrix versus a biphasic nonanimal stabilized hyaluronic acid filler after single injection in nasolabial folds. Dermatol Surg. 2013; 39(7): 1097-105.
7. Carruthers J, Carruthers A, Monheit GD, Davis PG. Multicenter, randomized, parallel-group study of onabotulinumtoxinA and hyaluronic acid dermal fillers (24-mg/ml smooth, cohesive gel) alone and in combination for lower facial rejuvenation: satisfaction and patient-reported outcomes. Dermatol Surg. 2010; 36 Suppl 4: 2135-45.
8. Malay S, Chung K. How to use outcome questionnaires: pearls and pitfalls. Clin Plast Surg. 2013 April; 40(2): 261–269.
9. Few J, Cox S, Paradkar-Mitragotri D, Murphy D. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015; 35(5): 589–599.
10. Shetty R. Outer circle versus inner circle: special considerations while rejuvenating an indian face using fillers. J Cutan Aesthet Surg. 2015; 8(3): 169-72.
11. Rohrich RJ, Pessa JE. The retaining system of the face: histologic evaluation of the septal boundaries of the subcutaneous fat compartments. Plast Reconstr Surg. 2008; 121: 1804–1809.
12. Schlager S, Rudell A. Sexual dimorphism and population affinity in the human zygomatic structure-comparing surface to outline data. Anat Rec (Hoboken) 2017; 300(1): 226-237.
13. Akinbiyi T, Othman S, Familusi O, Calvert C, Card EB, Percec I. Better results in facial rejuvenation with fillers. Plast Reconstr Surg Glob Open. 2020; 8(10): e2763.
14. Huggins RJ, Mendelson BC. Biologic behavior of hydroxyapatite used in facial augmentation. Aesthet Plast Surg. 2017; 41(1): 179-184.
15. Amin SP, Marmur ES, Goldberg DJ. Complications from injectable polyacrylamide gel, a new nonbiodegradable soft tissue filler. Dermatol Surg. 2004; 30(12): 1507-9.
16. Grover R.Optimizing treatment outcome with Restylane SubQ: the role of patient selectionand counselling. Aesthet Surg J. 2006; 26(1S): S18-21.
17. Few J, Cox SE, Paradkar-Mitragotri D, et Al. A Multicenter, Single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015; 35(5): 589-99.
18. van Loghem JAJ, Humzah D, Kerscher M. Cannula versus sharp needle for placement of soft tissue fillers: an observational cadaver study. Aesthet Surg J. 2017; 38: 73–88.
19. 33. Pavicic T, Frank K, Erlbacher K, et al. Precision in dermal filling: a comparison between needle and cannula when using soft tissue fillers. J Drugs Dermatol. 2017; 16: 866–872
20. Torres S. Curved cannulas for facial rejuvenation. IMCAS Congress, Paris, February, 2017, Poster Presentation.
21. Vedamurthy M, Vedamurthy A. Dermal fillers: tips to achieve successful outcomes. J Cutan Aesthet Surg. 2008; 1(2): 64–67.
22. Schreiber JE, Terner J, Stern CS, et Al. The boomerang lift: a three-step compartment-based approach to the youthful cheek. Plast Reconstr Surg. 2018; 141(4): 910-913.
23. Lorenc ZP, Lee JC. Composite volumization of the aging face: supra-periosteal space as the foundation for optimal facial rejuvenation. J Drugs Dermatol. 2016; 15(9): 1136-41.