Originals
Alessio Redaelli
International expert consensus on the use of AboBotulinum Toxin A (AboTA) for facial rejuvenation and primary hyperhidrosis
Keywords | Summary | Correspondence | References
Keywords
Abobotulinum toxin A, AboTA, Botulinum toxin A, hyperhidrosis, mimical muscles, mimics, rejuvenation
Schlüsselworte
Summary
Introduction: Recent developments in our understanding of facial ageing have led to a greater appreciation of the part played by dynamic wrinkles. Botulinum toxin is increasingly used to lessen hyperdynamic muscular activity and to rejuvenate the ageing face. Materials and method: A group of international experts convened to consider the literature and, in the light of their own clinical experience, discuss the optimal uses of abobotulinum toxin A (aboTA) for myomodulation. To assist doctors, the international expert group here presents consensus guidelines for the use of AboTA in various clinical indications. Discussion: To achieve optimal results, the clinician requires a detailed understanding of facial anatomy, correct dilution technique, injection procedure and aftercare. Conclusions: AboTA may be used to rejuvenate the face and other areas. AboTA treatment is effective, safe, and relatively easy to perform and has high patient satisfaction. Duration of action is up to 5 1/2 months.
Zusammenfassung
Redaelli A, Saromytskaya A, Rowland Payne C et al (2017) International expert consensus on the use of AboBotulinum Toxin A (AboTA) for facial rejuvenation and primary hyperhidrosis. CosMed 3(2): 70 – 80.
Redaelli A.a, Saromytskaya A b, Rowland Payne C.c., Manturova N. d, Battistella M.e, Saban Y.f, Panova O.g, Wollina U.h, Landau M. i, Atamanov V. j, Gavasheli L.k, Sanches E l, Gubanova E.m, Orlova O. n, Diaspro A.o, Kobaladze N.p, Reznik A.q, Lukyanau A.r, Sharova A. s, Zhaboeva S.t Holod O.u, Zhumatova G.v, Goltsova E.w, Soiher M. x, Chaikovskaya E.Y , Chebotareva J.Z, Parsagashvili E. ZZ
a Cosmetic Department, Visconti di Mondrone Medical Center, Milan, Italy
b Aestetical Medicine Clinica, Volgograd, Russian Federation
c The London Clinic, London, UK
d Chief Department Plastic and Reconstructive Surgery, Pirogova N.I.Moscow, Russian Federation
e Aesthetic Practisioner , Italy
f Nice, France
g Medical Centre “Eklan”, Moscow, Russian Federation
h Department of Dermatology and Allergology, Academic Teaching Hospital Dresden-Friedrichstadt, Dresden, Germany
i Dermatology, Wolfson Medical Center, Holon, Israel
j IRTC, “Eye Microsurgery” S.N. Fyodorov FSAI of the Ministry of Health Care, Novosibirsk, Russian Federation
k Clinic of Youth by Liya Gavasheli, Moscow, Russian Federation
l Medical Center of Cosmetology Correction EKLAN, Moscow, Russian Federation
m Clinic “Vallex-Med”, Moscow, Russian Federation
n I.M. Sechenov First Moscow State Medical University, Clinic of Nervous Diseases, A.Y. Kozhevnikov, Centre of Interdisciplinary Dentistry and Neurology, Moscow, Russian Federation
o Alberto Diaspro – Medico Estetico, Torina, Italy
p Clinic “Medclinic”,Saint-Petersburg, Russian Federation
q Medical Centre “ARclinic”,Saint-Petersburg, Russian Federation
r Belarusian State Medical University, Minsk, Belarus
s Center of Aesthetic Medicine “Chistye Prudy”, Moscow, Russian Federation
t Clinic of Youth and Beauty “SL”, Kazan, Russian Federation
u Center of Laser Aesthetics “Romital Clinic”, Kyiv, Ukraine
v Clinic of Aesthetic Medicine and Plastic Surgery “Armed”, Almaty, Kazakhstan
w LLC “Neo-clinic”, Tyumen, Russian Federation
x Center of Interdisciplinary Dentistry and Neurology, Moscow, Russian Federation
Y Institute of Plastic Surgery and Cosmetology, Moscow, Russian Federation
Z “Estelab” Clinic of Aesthetic Medicine, Moscow, Russian Federation
ZZ “Aestima Clinic” of Aesthetic Medicine, Saint Petersburg, Russian Federation
Introduction
In modern society, beauty canon is increasingly identified with perfect symmetry and important characters. Women and men have a self-evaluation fee that is reprogrammed from time to time. Common distinctive requirements are oval face, skin compactness and uniformity, eye magnetism, and lips’ appeal. Time is inexorably affecting every single of these beauty factors by marking the skin with wrinkles [1,2,] that develop even in younger persons, from habitual or dynamic muscular hyperactivity, as consequence of emotions or other stimuli. The severity and physiological changes of our body redesign the features of facial compartments, launching a big challenge to self-acceptance.
The advent of modulators for dynamic wrinkles enhancement has expanded rapidly by lengthening the time before resorting to surgery for facial rejuvenation [3].
Although the law rules differently the use of botulinum toxin in most countries of the world, the scientific community has universally recognized this product with a high security profile for the patient and after years of research identified parameters for a correct use even in those areas considered “off –label”.
One of the most used BTA toxins is Аbobotulinumtoxin A (AboTA). In Russia AboTA was approved in 1999 for neurological indications, in 2004 for aesthetic indications and in April 2009 FDA approved it also for therapeutic use. Very popular and most used among neurologists and ophthalmologists, each area of the three-thirds of the face that present muscle hyperkinetic activity can benefit from this type of treatment, starting with glabella, forehead, periorbital and perioral regions, from masseter to platysma for aesthetic and functional purpose too.
In some countries, mainly in Europe, AboTA is marketed under another trade name.
Materials and methods
In PubMed: Abobotulinumtoxin A. Abbreviation, AboTA
AboTA is not so widely used in aesthetic medicine as it is in neurology and so we have developed guidelines with the purpose of sharing knowledge, experience and expertise to help enhance the quality of service provided to patients by doctors using AboTA.
These guidelines for the use of AboTA are designed to help identify and/or specify:
– Characteristics and properties of AboTA
– Pre-treatment considerations
– Anatomical danger areas
– Technical considerations
– Post-treatment conclusions
– Aesthetic indications for “on label indications”
– Aesthetic indications for “off label indications” (according to the experience of the authors)
– Anticipated results
AboTA is present in the market in vials of 300 and 500 U. The chain of BTA joined with some non-toxic accessory proteins (NAPs) weighs 150 kDa. AboTA acts at the neuromuscular junction in the targeted muscle, thereby reducing muscular contraction. This lessens muscular strength and also lessens resting tone and so achieves temporary improvement in the appearance of dynamic lines.
The mechanism of action is simple. The exocytosis of acetylcholine into the synaptic gap is performed by a protein complex (SNARE) that allow the consequent activation of the muscle fiber [4]. Once injected, the toxin penetrates inside the cell for endocytosis, and in the presynaptic cytoplasm plays a proteolytic on the SNARE complex and then blocks the release of acetylcholine. By time, the nerve cell will synthesize and transport the SNARE complex’s proteins again to the presynaptic terminal. This is the reason why AboTA action can be considered reversible.
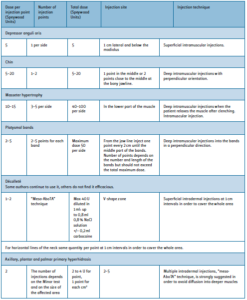
The authors conducted a search in Ovid MEDLINE, PubMed, Embase, and the Cochrane Library looking for “AbobotulinumtoxinA”, “facial rejuvenation” and “lines” and they carried out a systematic review of the more recent literature, specifically from January 2010 to October 2016 (Table 1).
By texts, contents and thanks to their long experience in the use of AboTA, they summarized the found data in specific guidelines to allow doctors to have a reference point for a good use of the product regarding immunological, safety and efficacy aspects.
All patients, without distinction of sex or ethnicity,can be successfully treated with AboTA in on-label areas [5-8].
AboTA can also be used in other “off-label” areas affected by ageing such as neck and chest [9, 10, 28, 29] or for the treatment of masseter hypertrophy [11], as well as in other indications like hyperhidrosis [12].
Crow’s feet:
Many studies have been conducted to assess the influence of the number of injections in this area for the distribution of the same amount of units. It has emerged that treating one side with a single injection of 36 U in the middle of central lateral at the ocular cantus does not show statistically significant differences in terms of results compared to three injections of 12 U each distributed along the same area [13].
Glabellar lines:
Other studies have been conducted to evaluate the efficacy of two different injective schemes for the treatment of glabellar wrinkles [14]. Specifically, the procerus and the two corrugators were injected in 3 points with 10 U per point and the results were compared with the same patterns to which two injective points (one on each side at 1 cm above the corrugator) of 10 U were added. The researchers pointed out that the two additional points with a larger number of units are irrelevant to the final result and that it didn’t improve efficacy.
Even the best dose in effect has been studied by comparing the results obtained by injecting respectively 20, 50 and 75 U into the glabellar area to improve the appearance of wrinkles. The most tolerated, effective and safe dose was judged at 50 U.
The important factor is that all patients treated with AboTA showed high satisfaction for the type of results obtained, declaring an improvement in the quality of social life throughout the time that AboTA was active [15].
Safety: The safety profile of AboTA used for facial rejuvenation is well established. Among thousands of patients treated no serious adverse events (AE) have been observed. All reported AEs were mild or moderate in severity and are usually caused by wrong technique [16-19, 30].
In addition to being well tolerated, the safety and efficacy of AboTA for glabellar wrinkles [4,5], universal “on label” treatment, is confirmed by many researchers in international scientific literature.
Numerous studies have been also conducted to analyze the different results based on the dilution of AboTA. Each dilution analyzed showed rapid and long-lasting efficacy, equal to injection pain [20].
Nowadays, after years of research, it is possible to compare the efficacy of different toxins in other areas of the face [21-31]. The efficacy and safety of AboTA injections is similar when used with a conversion factor of 1:2.5 – 1:3 compared with OnaTA or IncoTA [24].

Fig.: 2a – c: Depressor anguli oris (DAO) and depressor labii inferioris (DLI): 3rd area of caution.
Discussion
This expert group included dermatologists, plastic and maxillo-facial surgeons and aesthetic physicians. They gathered to discuss about recognized texts in literature found through online research and to share their own knowledge. Particular attention has been paid to the doses to be injected, to which specific points, with what dilutions, at which depth with emphasis on anatomical details, that are different for each individual.
The expert group reached a consensus on most issues with special recommendations for the use of AboTA in different anatomical areas.
The specialist’s advice to start with a set-up phase before treatment with AboTA that includes different points:
Pre treatment considerations
As in all branches of medicine:
- History, clinical examination and diagnosis are necessary steps before any treatment is considered.
- Absolute contraindications and relative contraindications should be excluded.
- Any bleeding tendency should be considered.
- Informed consent: Oral, and preferably written, informed consent is desirable.
- Medical Record: A medical record should be kept.
- Photography: Photography may be helpful.
Patients are treated in a reclining position, 30°. The skin of the patient is cleansed to remove residues, as make-up can result in post-operative complications [1, 12]. Particular attention and care is taken disinfecting the anatomical area to be treated.
Following a post-treatment protocol of skin care, according to some of the experts, produced better results and a decreased rehabilitation period after procedure.
For the treatment of very sensitive anatomical areas or in patients particularly sensitive to painful stimuli, topical anesthetic may be used.
Anatomical danger areas [18] (Anatomical study by Saban I.)
The anatomical details suggested by anatomical studies suggest the depth of injection and the exact injection site.
Glabellar area, 1st area of caution: Note that the procerus muscle runs from its deep bony origin on the nasal bones caudally to its insertion into the deep aspect of the skin in the glabellar area cephalically, superficial to and between the two frontalis muscles.
In the glabellar area, frontalis is always very superficial while the depressor muscles are deeper. For this reason, and to avoid the classical “Botox look”, it is important to inject deeply thereby only injecting the depressor muscles.
Crow’s feet inferior area, 2nd area of caution:
- The finger is placed just inferior to the caudal border of the zygomatic arch; the tip of the finger is blocked anteriorly by the body of the zygomatic bone (Fig. 1a)
- Just cephalic to the tip is located the bony origin of the zygomaticus major muscle [18]
- The zygomatic arch and bone have been drawn on the skin (Fig. 1b)
- The anterior border of the masseter m. is marked
- The orbicularis oculi (pars orbitalis) is represented, following the limits of the crow’s feet wrinkles
- The zygomaticus major muscle is drawn between its zygomatic bony insertion and the modiolus
- The depressor anguli oris (DAO) and depressor labii inferioris (DLI) muscles are already marked (Fig. 1b)
- The zygomatic area is shown, after resection of 3 first layers (Fig. 1c):
- 1° the skin
- 2° the malar subcutaneous fat pad
- 3° the orbicularis oculi layer
- The bony origins of zygomaticus major and minor muscles are exposed.
- The suborbicularis oculi fat (SOOF) (*) is the fat pad located in the prezygomatic space, which is just cephalic to the origins of these muscles
Depressor anguli oris (DAO) and depressor labii inferioris (DLI): 3rd area of caution:
- DAO muscle is a triangle with its base on the mandible (origin), its anterior border running perpendicularly upwards to the oral commissure (insertion) and its posterior border running obliquely downwards and backwards from lateral to oral commissure (Fig. 2a)
- DLI is a rectangle whose infero-posterior part lies deep to DAO. It originates from the mandible and inserts into the lateral half of the lower lip.
- The blue square, 3 cm wide, represents the area of the dissection
- The * represents the foramen of the trigeminal nerve 3rd branch (Fig. 2a)
- The DAO lies deep on the caudal border of the mandible where it originates and where it is covered by the subcutaneous fat; it becomes more superficial and inserts into the modiolus (Fig. 2b)
- The DLI and the orbicularis oris muscles have been resected (Fig. 2c)
- Note that the DLI caudal fibers are pass deep to the DAO as they run from their origin on the caudal border of the mandible. Its fibres are oblique cephalically and medially and pass deep to the OO muscle
- The exit point of the mandibular sensory nerve (Fig. 2a): its foramen is located where DAO overlaps superficial to DLI.
- Lateral to DAO, the inferior labial artery is dissected. (**)
Masseter and risorius muscles: 4th area of caution.
The lateral fibres of risorius originates in the preparotid fascia. Very near, but more deeply lie the fibres of masseter muscle. To avoid asymmetries of the smile, it is important to inject masseter deeply.
Knowledge and understanding of these 4 areas of caution is very important in order to avoid complications and bad results.
Summary of the recommendations of the international expert consensus – all areas
Group discussion:
The optimum interval between treatments is 4 months or more. Subsequent treatments follow the same scheme or are adapted to the current situation.
It is the unanimous opinion of the international expert consensus group that to use AboTA the operator requires a degree in medicine and surgery. Specialization in dermatology or plastic surgery is an advantage. Training in aesthetic medicine through accredited courses is helpful. The treatment should be performed in a clinical setting.
Conclusions
Innumerable scientific clinical studies and countless years of injector experience have demonstrated the efficacy and safety of AboTA as well as its action in terms of onset and duration.
Moreover, considering the annual cost of the treatment with AboTA instead of with other neuromodulators, it results lower [32].
The guidelines here presented by the international expert consensus group are based on our current knowledge. The guidelines reflect data obtained from reference scientific literature. Subsequent studies may lead to amendments or changes to these recommendations and/or to these conclusions of this document. Compliance with the guidelines guarantees neither treatment satisfaction nor a risk-free procedure. The data should always be analyzed and interpreted carefully, with proper critical analysis. All AboTA procedures should depend on the clinical assessment. The clinical experience and the clinical judgement of the doctor performing the treatment is much more important than any guidelines can ever be.
The use of AboTA raises ethical, cultural and legal considerations both for medical users and for manufacturers.
Address of Correspondence
Prof. Alessio Redaelli, M.D.
Via delle Repubblica 4
I-20098 San Giuliano Milanese
mail@docredaelli.com
Conflict of Interests
Redaelli A, Saromytskaya A, Panova O, Atamanov V, Kobaladze N, Gavashely L, Goltsova E, Sanches E, Gubanova E, Orlova O, Reznik A, Lukyanau A, Sharova A, Zhaboeva S, Holod O, Zhumatova G, Soikher M, Shelekhov S, Saromytskaya A are trainers and speakers for Ipsen company Landau M, Atamanov V, Gubanova E are trainers and speakers for Galderma Company Kobaladze N, Diaspro A, Zhaboeva S, Gavasheli L are trainers for Aptos Company Sanches E is medical adviser of the company Oftaderm Orlova O is trainer and speaker for Allergan, Martinex, Microgen and Merz Aesthetics Companies Reznik A is speaker and trainer for the Institute Hyalual Company Sharova A is trainer and speaker for Innovation and LG company Goltsova E is trainer and speaker for the Merz Aesthetics Companies Redaelli A, Zhumatova G are trainers and speakers for Filorga company Rowland Payne C is a key opinion leader for Venn and an occasional speaker for Ipsen & Sesderma. Battistella M is trainer and speaker for General Project, Wavemed, Attiva, Venus Concept Companies
References
1. Redaelli A, Braccini F (eds).: Facial aging. Medical, surgical and odontostomatological solutions for mid & inferior part of the face. Florence: Officina Editoriale Oltrano: 2012.
2. Wollina U, Rowland Payne C (2010) Aging well-the role of minimally invasive aesthetic dermatological procedures in women over 65. J Cosmet Dermatol 9(1): 50-8.
3. Wollina U. Botulinum toxins: uses in medicine. Cosmetic medicine and surgery. Ed: Andrè P, Haneke E, Marini L, Rowland Payne C. Boca Raton, FL: CRC Press, pp 547-5.
4. Wollina U (2008) Botulinum toxin: Non-cosmetic indications and possible mechanisms of action. J Cutan Aesthet Surg 1(1): 3-6.
5. Cohen JL, Scuderi N (2017) Safety and patient satisfaction of abobotulinumtoxinA for aesthetic use: a systematic review of clinical studies. Aesthet Surg J 5: 37(suppl_1): S32-S44
6. Schlessinger J (2014) Long-term safety of abobotulinumtoxinA for the treatment of glabellar lines: results from a 36-month, multicenter, open-label extension study. Dysport Study Group. Dermatol Surg. 40(2): 176-83.
7. Lorenc ZP (2013) A review of AbobotulinumtoxinA (Dysport). Aesthet Surg J 33 (1 Suppl):13S-7S.
8. Fabi SG (2013) A two-center, open-label, randomized, split-face study to assess the efficacy and safety of one versus three intradermal injection sites of abobotulinumtoxinA in the treatment of lateral periocular rhytides. J Drugs Dermatol 12(8): 932-7.
9. Erickson BP (2015) The role of neurotoxins in the periorbital and midfacial areas. Facial Plast Surg Clin North Am 23(2): 243-55.
10. Klein FH (2014) Lower facial remodeling with botulinum toxin type A for the treatment of masseter hypertrophy. An Bras Dermatol. 89(6): 878-84.
11. Redaelli A (2011) Botulinum toxin A in bruxers: one year experience. Saudi Med J 32(2): 156-158.
12. Redaelli A (2014) BTxA in aesthetic Medicine, for hyperhidrosis and in odontostomatology. Basic Principles and clinical practice. Second revised edition, OEO Firenze.
13. Kiripolsky MG, Goldman MP (2011) Safety and efficacy of administering abobotulinumtoxinA through a single injection point when treating lateral periocular rhytides. J Cosmet Dermatol 10(3): 232-234.
14. Rzany B (2006) Efficacy and safety of 3- and 5-injection patterns (30 and 50 U) of botulinum toxin A (Dysport) for the treatment of wrinkles in the glabella and the central forehead region. Arch Dermatol 142(3): 320-6.
15. Hexsel D (2013) Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: A randomized, phase IV clinical trial. J Drugs Dermatol 12(12): 1363-7.
16. Kim BW (2014) Adverse events associated with botulinum toxin injection: a multidepartment, retrospective study of 5310 treatments administered to 1819 patients. J Dermatolog Treat 25(4):331-6.
17. Yin-Shuo Chang (2015) Nonallergic eyelid edema after botulinum toxin type A injection. Case report and review of literature. Medicine (Baltimore). 94(38): e1610.
18. Saban I (2016) Anatomie du visage et du cou en chirurgie et cosmetology. Elsevier Masson.
19. Wollina U (2005) Managing adverse events associated with botulinum toxin type A: a focus on cosmetic procedures. Am J Clin Dermatol 6(3): 141-50.
20. Punga AR, Alimohammadi M, Fagrell D, Nyberg F, Rees D, Wong C (2016) A randomized, comparative study to evaluate efficacy and safety of 2 injection volumes of abobotulinumtoxinA in treatment of glabellar lines. Dermatol Surg 42(8): 967-76.
21. Frevert (2015) Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. J Drugs Res Derm 15(1): 1-9.
22. Kassir R, Kollurur A, Kassir M (2013) Triple-blind, prospective, internally controlled comparative study between abobotulinumtoxinA and onabotulinumtoxinA for the treatment of facial rhytids. Dermatol Ther 3(2): 179-89.
23. Yu KC, Nettar KD, Bapna S, Boscardin WJ, Maas CS (2012) Split-face double-blind study comparing the onset of action of onabotulinumtoxinA and abobotulinumtoxinA. Arch Facial Plast Surg 14(3): 198-204.
24. Scaglione F (2016) Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins (Basel) 2016 8(3).
25. Naumann M (2013) Immunogenicity of botulinum toxins. J Neural Transm (Vienna) 120(2): 275-90.
26. Rubin MG Cox SE, Kaminer MS, Solish N (2014) Correcting age-related changes in the face by use of injectable fillers and neurotoxins. Semin Cutan Med Surg 33(4 Suppl): S81-4.
27. Redaelli A (2012) Minimally invasive procedures for nasal aesthetics. J Cutan Aesthet Surg 25(2): 115-20.
28. Rowland Payne CME (2012) Multi-mini botulinum. Chapter in: Botulinum toxin A in aesthetic medicine. Eds. Redaelli A, Braccini F. Officina Editoriale Oltrarno S.r.l – Firenze, 185-198.
29. Rowland Payne CME (2016) Cosmetic botulinum toxin treatment. In: Andre P, Haneke E, Marini L & Rowland Payne CME, editors. Cosmetic Medicine & Surgery. 1st ed. Taylor & Francis-CRC Press (London); p. 557-80.
30. Rowland Payne CME (2016) Complications & pitfalls of cosmetic botulinum. In: Andre P, Haneke E, Marini L & Rowland Payne CME, editors. Cosmetic Medicine & Surgery. 1st ed. Taylor & Francis-CRC Press (London); p. 581-89.
31. Rowland Payne CME (2017) Cosmetic dermatology of the nose and medical rhinoplasty. Aesthetic Surgery Techniques – A Case Based Approach. Elsevier. In press.
32. Ravi Jandhyala
33. Jandhyala R (2012) Effectiveness of type A botulinum toxins for aesthetic indications and their relative economic impact. J Plast Reconstr Aesthet Surg 65(6): 720–731.